From "Unknown Market" to FDA Application
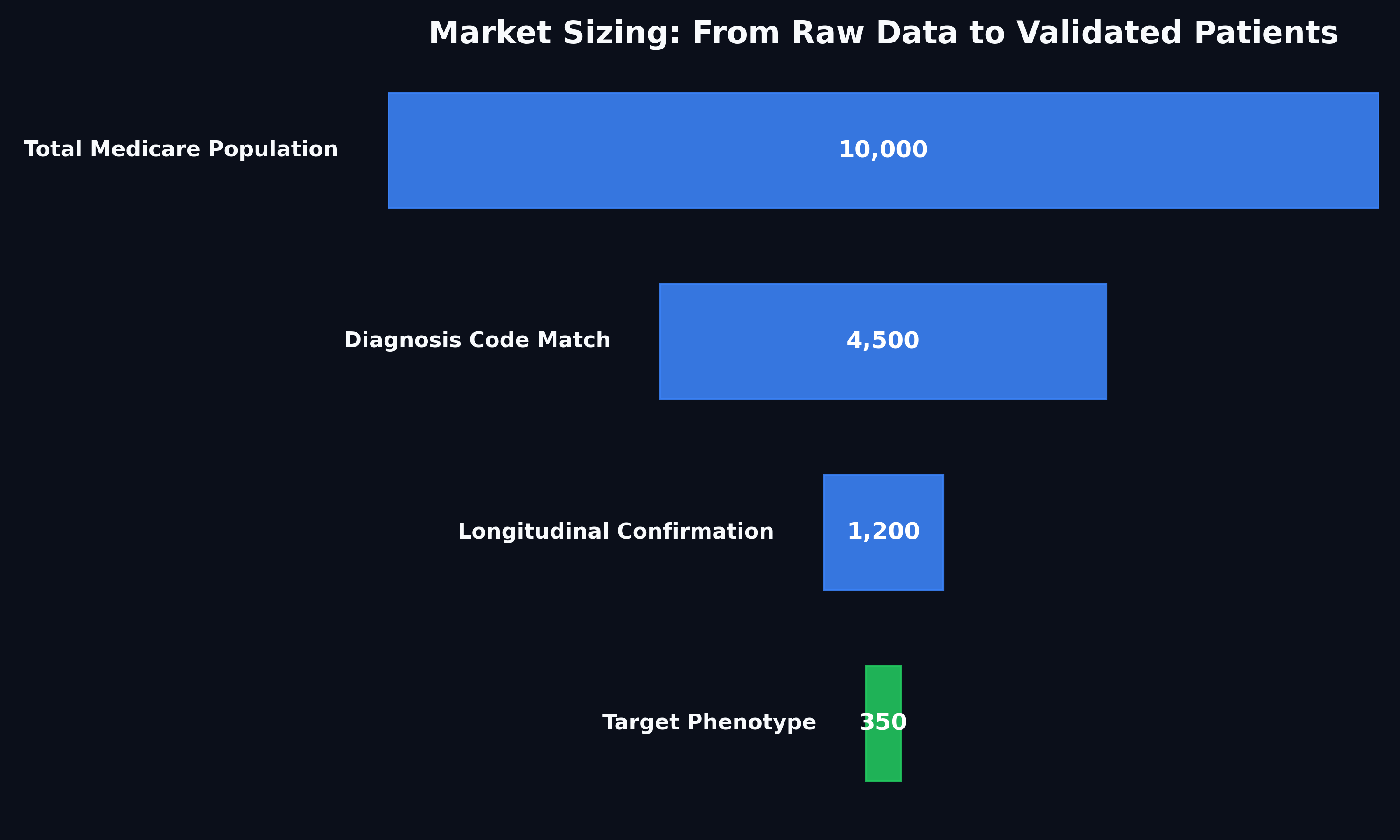
A pharmaceutical client was preparing an FDA application for a new treatment targeting a rare disease. A critical component of the submission was defining the Total Addressable Market (TAM). However, standard ICD codes failed to capture the specific patient phenotype, leaving the client with no reliable data.
We executed a deep-dive analysis of a large-scale Medicare purchase dataset. Instead of relying on generic codes, we applied:
Complex Filtering Logic: Aligned operational claims data with strict academic clinical definitions.
Longitudinal Tracking: Followed patient histories to confirm diagnosis patterns and rule out false positives.
Strategic Confidence
Provided a scientifically defensible patient count, unlocking investment for the drug's FDA application launch.
Actionable Commercial Output
Delivered validated revenue projections and a ranked list of healthcare providers most likely to adopt the treatment — ready for the commercial team on day one.